A molecular gatekeeper that controls protein synthesis

The “Molecular Octopus” Revealed: How ETH Zurich’s Discovery Rewrites the Rules of Life and Cancer Treatment
Brainx Perspective
At Brainx, we believe that the next frontier in medicine lies not just in understanding what our cells create, but how they manage the chaos of creation. This breakthrough from ETH Zurich highlights a monumental paradigm shift: biological precision is not accidental; it is orchestrated. By identifying the “master conductor” of protein synthesis, we are finally seeing the invisible hands that guard our genetic health, opening doors to therapies previously thought impossible.
The News: The Conductor of the Cellular Symphony
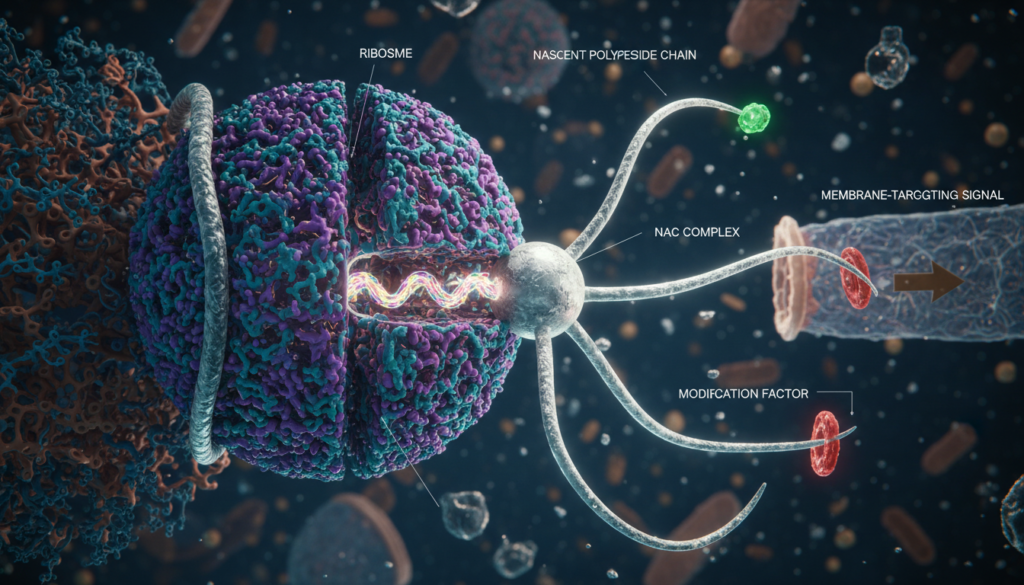
The human cell is often described as a bustling metropolis, but until recently, scientists struggled to understand how the traffic within this city was managed without descending into chaos. New research from ETH Zurich, led by Professor Nenad Ban, has provided the answer. They have mapped the structure and function of the Nascent Polypeptide-Associated Complex (NAC), a molecular machine that ensures proteins are built, modified, and transported with near-perfect accuracy.
Using advanced imaging technology, the team revealed that NAC acts as a “molecular octopus,” sitting at the exit of the ribosome (the cell’s protein factory) to catch and process every new protein the moment it is born.
Key Findings and Mechanisms:
- The “Octopus” Architecture: Through Cryo-Electron Microscopy (cryo-EM), researchers visualized the NAC complex in unprecedented detail. It features a central globular “body” and two flexible “arms.” One arm anchors the complex to the ribosome, while the other swings dynamically to recruit specific enzymes.
- The First Responder: As a new protein chain (polypeptide) emerges from the ribosome, the NAC is the first point of contact. It creates a protected environment, preventing the vulnerable chain from misfolding or clumping together, which is a precursor to neurodegenerative diseases like Alzheimer’s.
- Orchestrating Chemical Upgrades: Proteins are rarely ready for use immediately. They require chemical modifications. The NAC acts as a docking station for essential enzymes—specifically Methionine Aminopeptidase (MAP) and the NatA complex. These enzymes “trim” and “tag” the new protein to ensure stability.
- The Traffic Controller (SRP Interaction): The NAC regulates the Signal Recognition Particle (SRP). It effectively screens proteins; if a protein is meant for the cell membrane, NAC allows the SRP to bind. If not, it blocks the SRP. This prevents “traffic jams” and ensures proteins are not sent to the wrong cellular address.
- The Epigenetic Guardian (New Discovery): Perhaps the most shocking discovery is NAC’s role in Histone processing. Histones are the spools around which DNA is wound. The study found that NAC recruits the specific enzymes needed to modify histones. Without this, DNA compaction fails, leading to genomic instability—a hallmark of cancer.
Deep Dive: Why This Rewrites the Textbooks
To fully grasp the magnitude of this discovery, we must look at the specific implications across three major fields of science and medicine.
1. The Cancer Connection: Targeting the “Factory Manager”
Cancer cells are defined by rapid, uncontrolled growth. To sustain this, they must produce proteins at a speed that far exceeds that of a normal cell. This creates a high dependency on the machinery that manages protein quality—specifically the NAC.
- The Achilles’ Heel: Because cancer cells are “addicted” to high-speed protein synthesis, they heavily rely on NAC to prevent the accumulation of toxic waste.
- New Therapeutic Strategy: If researchers can develop drugs that inhibit the NAC’s “arms” from recruiting enzymes, they could induce a catastrophic buildup of misfolded proteins specifically within cancer cells. This would trigger the cancer cell to self-destruct (apoptosis) while potentially sparing slower-growing healthy cells.
2. The Genomic Link: NAC and DNA Architecture
The link between NAC and histones is a revelation. DNA is approximately two meters long and must be packed into a nucleus only micrometers wide. It does this by wrapping around histone proteins.
- The Gatekeeper of Genes: If histones are not processed correctly (a job now known to be managed by NAC), the DNA cannot be compacted properly.
- Epigenetic Errors: Improper compaction leads to genes being turned “on” or “off” at the wrong times. This suggests that NAC is not just a protein manager, but a guardian of epigenetics. Modulating NAC could offer a way to “reset” the corrupted genetic instructions found in tumors.
3. The Power of Cryo-Electron Microscopy
This discovery was only possible due to the “resolution revolution” in biology. Traditional X-ray crystallography requires molecules to be frozen in rigid crystals. However, the NAC is dynamic—it moves and swings its arms.
- Freezing Motion: Cryo-EM allowed the ETH Zurich team to flash-freeze the complex in its natural state.
- Molecular Movies: They reconstructed the NAC in various stages of motion, effectively creating a “molecular movie” that showed exactly how the flexible arms swing to fetch enzymes. This dynamic view of biology is replacing the static models of the past.
Why It Matters (Conclusion)
This development bridges the gap between atomic structure and clinical reality. For the common man, it promises a future where cancer therapies are laser-targeted, attacking the “supply chain” of the tumor rather than bombing the whole body. By decoding the “conductor,” science has moved beyond merely listening to the music of life—we are now learning how to tune the instruments to prevent the dissonance of disease.
Extended Technical Analysis: The Bio-Engineering Implications
(A deeper look for industry professionals and enthusiasts)
The Evolutionary Masterpiece The NAC complex represents a significant evolutionary leap. While bacteria possess a simpler version called the “Trigger Factor,” the eukaryotic NAC is far more sophisticated. Its multi-armed structure evolved to handle the immense complexity of human cells, where proteins must be sorted into various organelles (nucleus, mitochondria, ER). This complexity is what allows for multicellular life, but it is also what makes human cells vulnerable when the machinery breaks down.
Synthetic Biology and Industrial Production Beyond health, this research is a goldmine for the biotechnology sector.
- Optimizing “Cell Factories”: Industries use bacteria and yeast to produce insulin, antibodies, and enzymes. A major bottleneck is that these cells often get “stressed” and produce misfolded (useless) proteins.
- Engineering NAC: By genetically enhancing the NAC complex in these industrial cells, scientists could create “super-producers” that churn out higher yields of perfect proteins. This could lower the cost of life-saving drugs and bio-materials significantly.
The Proteostasis Network The study emphasizes that the ribosome does not work alone. It is the center of a “Proteostasis Network.” The NAC is the hub that connects the synthesis machinery (ribosome) with the folding machinery (chaperones) and the degradation machinery (proteasomes). Understanding this network is crucial for treating age-related diseases like Parkinson’s, which are essentially failures of proteostasis. If we can artificially boost NAC activity in aging cells, we might be able to slow down the cellular accumulation of waste that leads to degeneration.
Final Verdict The work by Professor Ban and his team at ETH Zurich is a functional blueprint for the machinery of life. It moves the field of molecular biology from a static “picture” to a dynamic “video,” revealing that the flexible, disordered parts of proteins are just as important as the rigid ones. As we move forward, the NAC complex will likely become a primary target for the next generation of small-molecule drugs and epigenetic therapies.



Leave a Reply